Institutional Review Board (IRB) Overview
Welcome to the Southern California Brainspotting Institute’s Institutional Review Board (IRB). All research conducted by the Southern California Brainspotting Institute must be approved and monitored by the IRB.
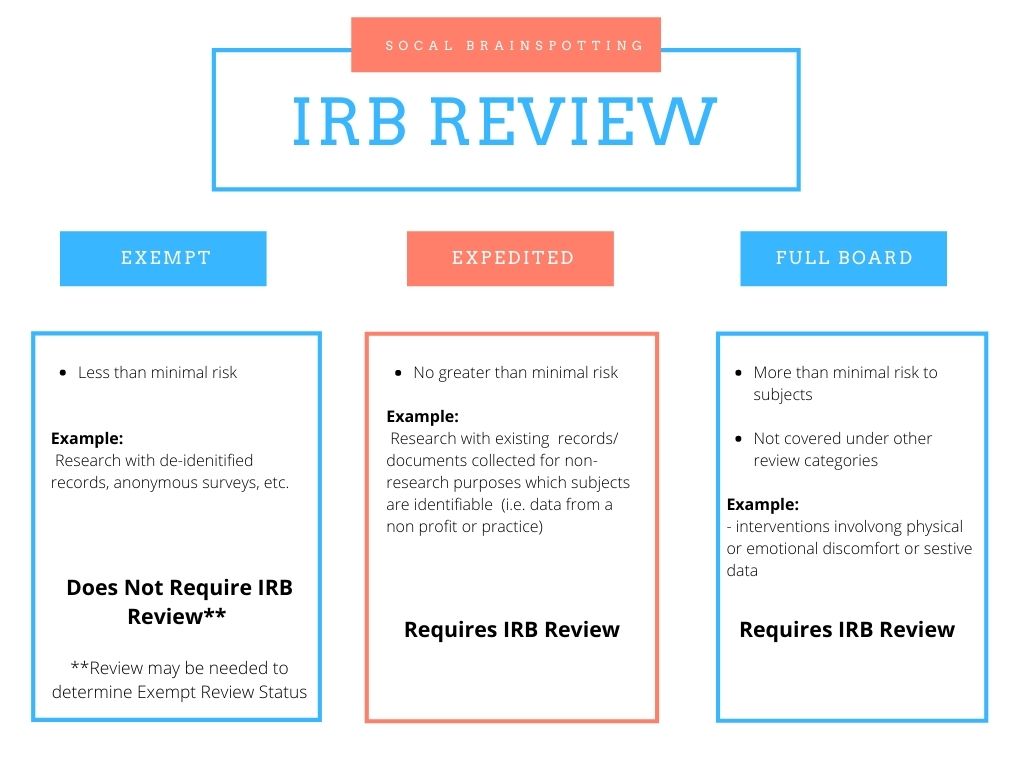
The Role of an IRB
United States federal regulations require that all research conducted with human subjects be subject to certain rules to assure subjects are treated ethically and safely. These regulations, known as “Common Rule” and outlined in 45 CFR 46, were created decades ago to combat human rights abuses in research studies.
Common Rule is a very large and complex set of regulations. Therefore, in order to assure compliance all affected research must be reviewed and approved by an Institutional Review Board (IRB) registered and in good standing with the Office of Human Research Protections at the United States Department of Health and Health Services.
Applicable Research
All research conducted with or at organizations receiving federal funding or associated with federal agencies must have their research approved by an IRB. This is true across the board: be it conducted at an institution or done privately, by a freelance scientist or independently; if it is medical or social/behavioral ; if it is at a federally sponsored institute but the project does not receive any funding specifically, and even with informed consent or waivers. Virtually any institute involving itself in research will have and require approval from an IRB, even if they are not funded federally.
In the case of not being associated with the government, IRB Review is not required, but federal regulations regarding the welfare of human research subjects are still applicable and are just as complex, having hundreds of sections and subdivisions.
Conducting research that violates human research ethics legislation is a federal offense and violators may be subject to fines and even imprisonment. Further, IRB approval is a strict, nonnegotiable requirement for all legitimate forms of publication and funding. This approval must be granted before any research is conducted, including recruitment or advertisement, and cannot be retroactively granted for research already conducted in part or in whole without approval.
Therefore, it is generally presumed across the research community that IRB approval is a requirement for all forms of human research for all researchers, as without it, publication and funding are impossible and it possesses immense legal liability.
Non-Applicable Research
In order to be considered human research, the survey must be conducted on humans – analyzing specifically their physical or mental characteristics on an individual level – and meet the definition of research – acquiring data to be analyzed and used to contribute to generalizable knowledge. Chart 01 provided at this website run by the HHS provides a helpful flowchart to determine if activity is considered human research.
Therefore, unless they are being used to analyze the human psyche and contribute to the general knowledge beyond those they interview, the following are not considered human research:
- Organizations conducting surveys or interviews about people’s opinions of products, things and policies and not themselves or their personal information (such as an opinion poll for an elected official)
- Surveys or interviews conducted about organizations to help improve services (customer satisfaction surveys)
- Journalist interviews or writing biographies about a specific individual
- Analyzing publicly accessible data (such as census data or labor statistics)
One should still be very cautious when assuming their research falls into the above criteria. Always check with an IRB before deciding you do not need review.
Important Notes on Secondary Review
A common misconception is that if you are not directly interviewing individuals, you can use data without IRB approval. This is only true in a small number of circumstances and must meet the below criteria.
- All direct identifiers (names, addresses, etc) are removed from the data set
- All indirect identifiers that could be used to deduce the identity of the individual are removed
- The information contained does not pose significant risk to the individuals if made publicly known
- The data has been made available from another survey/study compliant with Common Rule provisions
As with the above, never assume that research is exempt from review for broadly fitting into the above categories without first checking with an IRB.
Getting IRB Approval
Most institutions conducting human research have their own IRB to approve research projects proposed by their affiliates. For those that do not, IRBs at other institutions can enter into written agreements to review and approve studies at the institution if needed.
Further, independent IRBs not affiliated with an institution exist to approve research from independent or freelance researchers. While they generally charge a few, their approval is considered just as legitimate in satisfying the requirements for review in Common Rule.
Each IRB will request documents specific to their processes, but on a whole, they will require the following:
- A detailed proposal of the research project, what it seeks to research, what it anticipates finding and its impact
- Certificates of completion for required human research ethics training for the Principal Investigator and Research Aides/Staff
- Copies of the informed consent documents used in the research project, which should include
- A description of everything the subject will be asked to do
- An explanation that they may stop participating at any time
- An outline of any risks or benefits posed to them
- Information on how their information will be used, distributed and kept confidential
- Contact information on who to contact for concerns or problems.
- Copies of all surveys/questionnaires to be distributed or scripts for all interviews/experiments/observations to be conducted
- An outline of how subjects are selected for the study
- Written agreements for any sites the research will be conducted at
- Documentation of all grant money, budgets and payment plans and disclosures of any conflicts of interest or financial interests in the research on the part of the investigator, staff/aides or funders
- Copies of recruitment plans, advertisements and enrollment forms
- Other forms and affirmations as required by each organization
It is important to check with an IRB regarding their guidance for training and requirements for documents and study design before submitting to reduce the chances of needing to submit multiple proposals.
The approval process can be lengthy – some boards will meet once per month or less, and training and preparation of proposal materials might take time. These are important considerations in assigning timelines.