Proposal Submission
All Southern California Brainspotting Institute members wishing to conduct research are expected to submit a RP Form 1 – Proposal Factsheet. Please allow ten business days to process reviews. Additional time for review or supplementary forms may be requested.
Before opening the form, please take a minute to examine the ethics criteria the IRB will be using to review your study on the Resources and Training page. Also, click here to review the Principle Investigator Handbook which explains much of the terminology and expectations of a researcher. Finally, review the checklist below to make sure you have on hand everything you will need to complete the form.
Please click the link below to be taken to the online RP Form 1. Email IRB@SoCalBrainspotting.org if you have any issues or concerns.
Research Proposal Form 1 – Proposal Factsheet
If you are not able to access the form, please click here to download a PDF version. Send the completed form to IRB@SoCalBrainspotting.org along with all required files.
Materials needed to complete the form
- Up -to-date IRB ethics credentials for the Principle Investigator and all research team members. Email IRB@SoCalBrainspotting.org to receive instructions on accessing IRB ethics credential trainings.
- Sample instruments, including interview scripts, survey instruments and experiment protocols, that will be used in the research.
- A copy of your proposed informed consent form.
- If the research involves funding or payment, a budget will be required, along with invoices and direct deposit information if relevant.
- If research involves reliance or cooperation with another institution, please submit a letter of intent from the prospective secondary institution(s) complete with contact information to the designated liaison(s). Please see the Reliance page for my details.
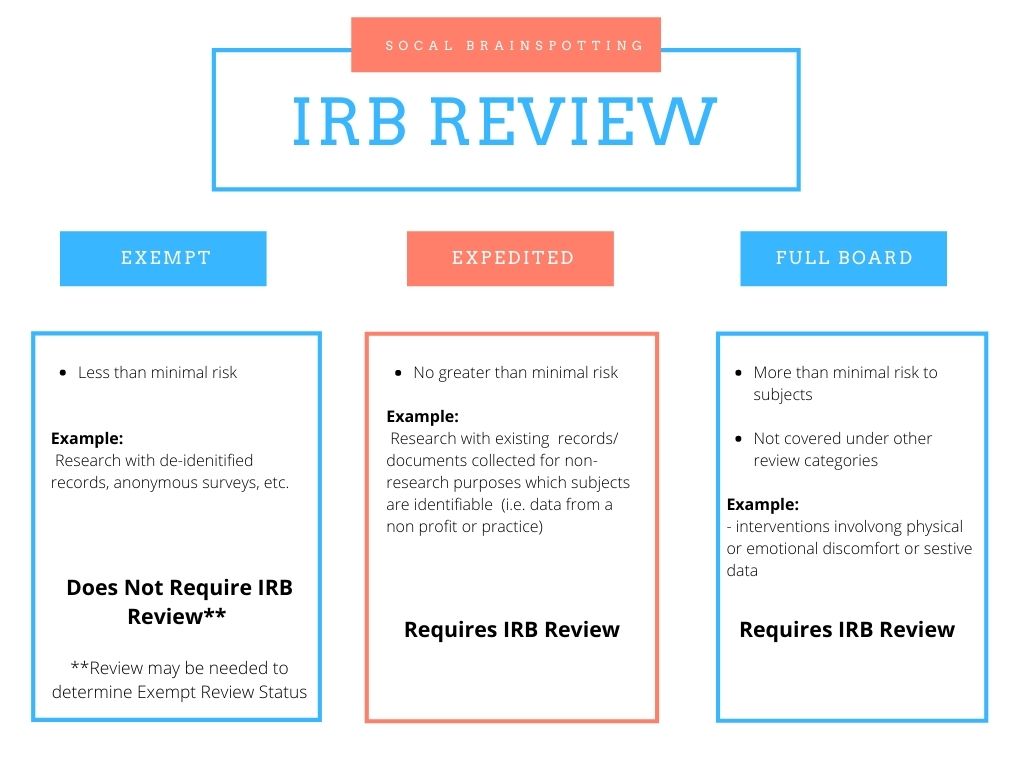
Types of Approval
Submissions approved by the IRB will be given one of three levels of approval, as outlined below.
Timeline
Once submitted, you can expect the following timeline.
- Allow for ten days for your submission to process. After ten days, you can expect a confirmation of receipt sent to the email you provided in the request, which will either notify you of what date the proposal will be discussed by the IRB or what additional forms or information is needed.
- If the submission is accepted, it will be discussed at the next IRB meeting, which occur monthly, for approval. If you are requested to be in attendance, you can expect to be notified at least five days prior. Meetings are held by teleconference.
- If your submission is approved, you can expect within seven days of the IRB meeting to receive a Notice and Terms of Approval, which will also detail Continuing Review. Please visit the Continuing Review page for more information.
- You can then begin research, and will be required to submit compliance forms back as detailed in your notice and terms of approval.
- If the request is denied, an explanation will be provided, including which, if any, amendments would be needed for approval.
Post-approval modifications
If it becomes necessary to modify the study protocols after approval, it is required to submit a CR3 Modification Request form to IRB@SoCalBrainspotting.org for approval. This is also required if there are any adverse events or complaints related to the study. It is the responsibility of the Principal Investigator to report modifications, adverse events or complaints to the IRB in a timely manner. Failure to do so may result in corrective action.
For more information on what requires a modification request and on corrective action, please visit the Continuing Review page.



